|
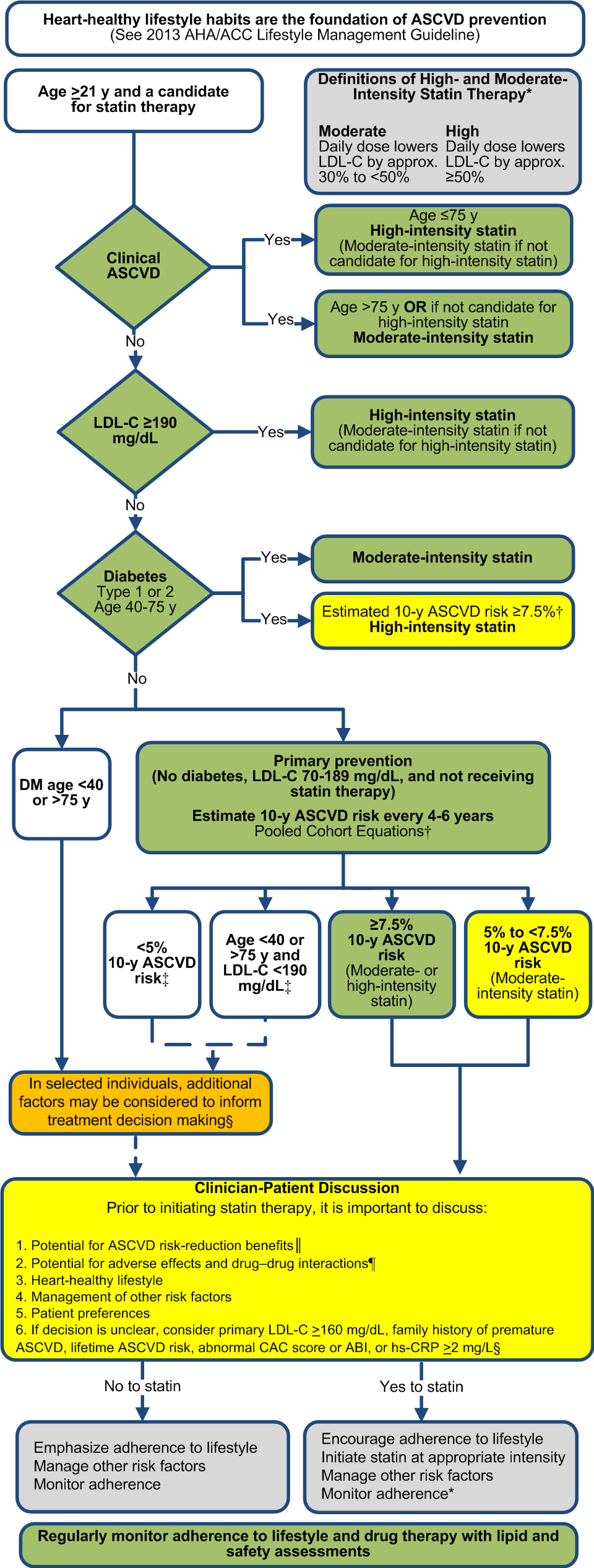
A. Heart-healthy lifestyle habits should be encouraged for all individuals.
|
|
B. The appropriate intensity of statin therapy should be initiated or continued:
|
|
1.
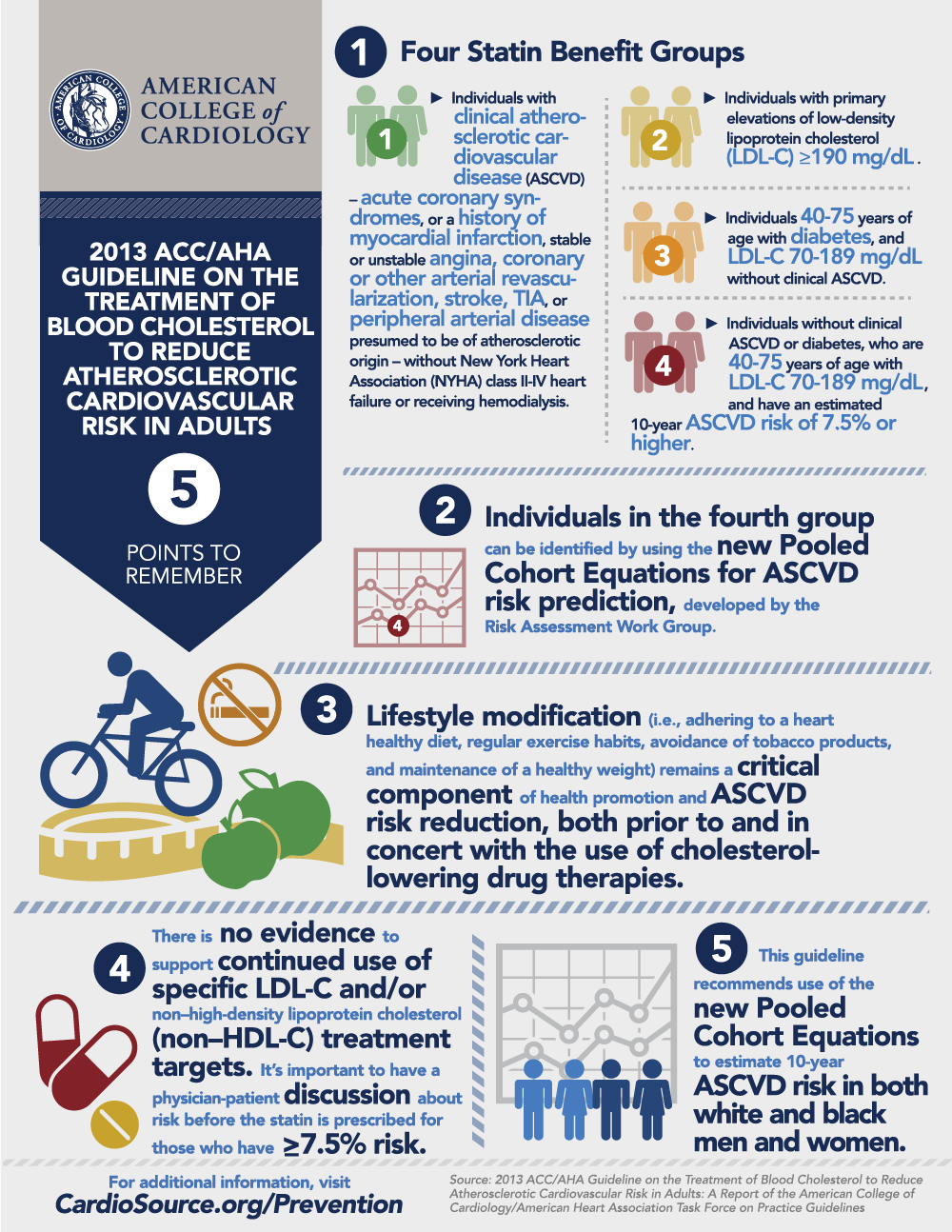
Clinical ASCVD*
|
|
a. Age ≤75 y and no safety concerns: High-intensity statin
|
I
|
A
|
|
b. Age >75 y or safety concerns: Moderate-intensity statin
|
I
|
A
|
|
2.
Primary prevention – Primary LDL-C ≥190 mg/dL
|
|
a. Rule out secondary causes of hyperlipidemia
|
|
|
b. Age ≥21y: High-intensity statin
|
I
|
B
|
|
c. Achieve at least a 50% reduction in LDL-C
|
IIa
|
B
|
|
d. LDL-C lowering nonstatin therapy may be considered to further reduce LDL-C
|
IIb
|
C
|
|
3.
Primary prevention - Diabetes 40-75 years of age and LDL-C 70-189 mg/dL
|
|
a. Moderate-intensity statin
|
I
|
A
|
|
b. Consider high-intensity statin when ≥7.5% 10-y ASCVD risk using the Pooled Cohort Equations†
|
IIa
|
B
|
|
4.
Primary prevention – No diabetes 40-75 years of age and LDL-C 70-189 mg/dL
|
|
a. Estimate 10-y ASCVD risk using the Risk Calculator based on the Pooled Cohort Equations† in those NOT receiving a statin; estimate risk
every 4-6 y
|
I
|
B
|
|
b. To determine whether to initiate a statin, engage in a clinician-patient discussion of the potential for ASCVD risk reduction, adverse
effects, drug–drug interactions, and patient preferences.
|
IIa
|
C
|
|
c. Re-emphasize heart-healthy lifestyle habits and address other risk factors.
|
|
|
i. ≥7.5% 10-y ASCVD risk: Moderate- or high-intensity statin
|
I
|
A
|
|
ii. 5 to <7.5% 10-y ASCVD risk: Consider moderate-intensity statin
|
IIa
|
B
|
|
iii. Other factors may be considered‡: LDL-C ≥160 mg/dL, family history of premature cardiovascular disease, hs-CRP ≥2.0
mg/L, CAC score ≥300 Agaston units, ABI <0.9 or lifetime ASCVD risk
|
IIb
|
C
|
|
5.
Primary prevention when LDL-C <190 mg/dL and age <40 or >75 y, or <5% 10-y ASCVD risk
|
|
a. Statin therapy may be considered in selected individuals‡
|
IIb
|
C
|
|
6.
Statin therapy is not routinely recommended for individuals with NYHA class II-IV heart failure or who are receiving maintenance
hemodialysis
|
|
C.
Regularly monitor
adherence to lifestyle and drug therapy with lipid and safety assessments.
|
|
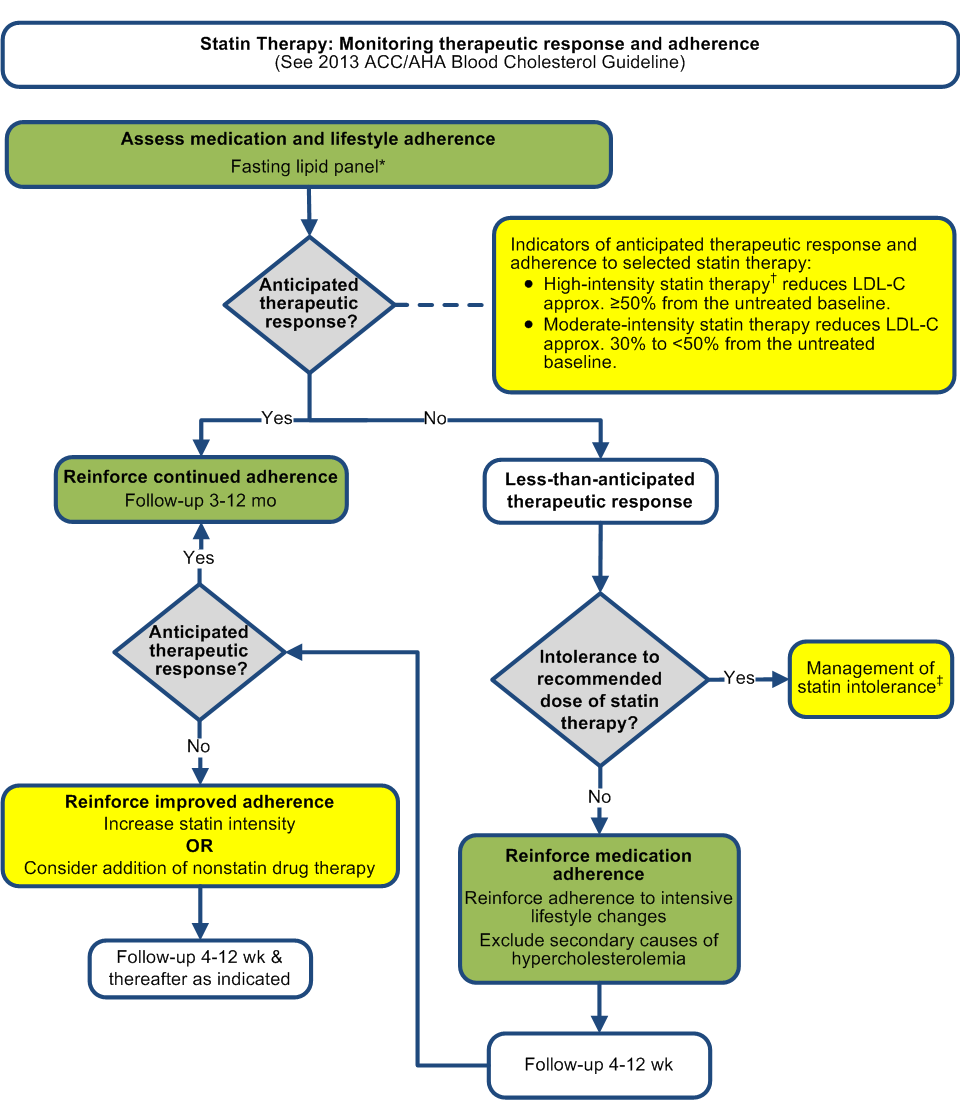
Assess adherence, response to therapy, and adverse effects within 4-12 wk following statin initiation or change in therapy.
|
I
|
A
|
|
a. Measure a fasting lipid panel
|
I
|
A
|
|
b. Do not routinely monitor ALT or CK unless symptomatic
|
IIa
|
C
|
|
c. Screen and treat type 2 diabetes according to current practice guidelines. Heart-healthy lifestyle habits should be encouraged to
prevent progression to diabetes
|
I
|
B
|
|
d. Anticipated therapeutic response approximately ≥50% reduction in LDL-C from baseline for high-intensity statin and 30% to <50% for
moderate-intensity statin
|
IIa
|
B
|
|
i. Insufficient evidence for LDL-C or non–HDL-C treatment targets from RCTs
|
|
|
ii. For those with unknown baseline LDL-C, an LDL-C <100 mg/dL was observed in RCTs of high-intensity statin therapy
|
|
|
e. Less than anticipated therapeutic response:
|
|
i. Reinforce improved adherence to lifestyle and drug therapy
|
I
|
A
|
|
ii. Evaluate for secondary causes of hyperlipidemia if indicated
|
I
|
A
|
|
iii. Increase statin intensity, or if on maximally-tolerated statin intensity, consider addition of nonstatin therapy in selected high-risk
individuals§
|
IIb
|
C
|
|
f. Regularly monitor adherence to lifestyle and drug therapy every 3-12 mo once adherence has been established. Continued assessment of
adherence for optimal ASCVD risk reduction and safety.
|
I
|
A
|
|
D. In individuals intolerant of the recommended intensity of statin therapy, use the maximally-tolerated intensity of statin.
|
I
|
B
|
|
1. If there are muscle or other symptoms, establish that they are related to the statin
|
IIa
|
B
|
|
2. For specific recommendations on managing muscle symptoms (see Statin Safety Recommendations)
|